The medical and healthcare sectors are critical to maintaining and improving our way of life. Part of the reason the medical field takes years of additional schooling and training is that it involves complex procedures and machines. Doctors and nurses not only have to learn life-saving surgeries but also how to operate the medical devices that assist during the procedures. This is why user interface design is so critical to the medical industry.
A good user interface design is not just nice to have – an elegant interface can save lives. Good user interface design can save time during training and procedures as the doctors and technicians don’t have to fumble around with confusing buttons. Medical devices built with user interface in mind tend to align with healthcare regulatory requirements, create solutions, and offer optimal functionality to satisfy doctor and technician needs.
An aesthetically pleasing design appeals to both the buyer—a medical device company—and the user—a doctor, nurse, or technician—increasing the potential to capture a larger market share for your product. That is why we approach medical device design from a holistic perspective rather than as an isolated part of the production process. It creates optimal opportunities for success—implementing precision at every stage—from concept through innovation to market.
Benefits of User Interface Design on Medical Devices
It takes significant effort to produce the right healthcare solution that will meet user needs. The solution must have strong features that capture needs and simplify interaction with the machine.
To achieve this, you need to hire a robust team who can design and manufacture a user interface design that meets all your needs. Butler Technologies’ skilled team is equipped to handle all your design needs, from napkin sketches or rough concepts to prototypes and into production.
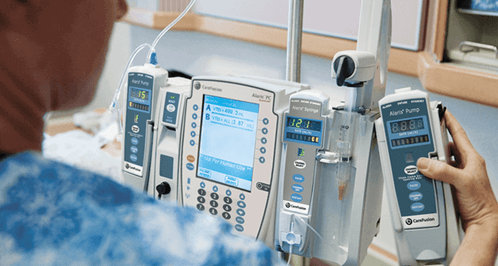
5 Key Reasons Why You Need the Right User Interface Design for Your Medical Device:
1. Quality
Quality is the utmost important factor if you want your product to stand the test of time. Therefore, this is an extremely key factor when considering a partner for your project. Innovation is important, but quality is still the foremost factor. Superior quality pays dividends down the road and requires investing in the right personnel, systems, and processes.
2. Adherence to Specifications and Requirements
Medical devices have to adhere to rigorous standards and testing. In some cases, a medical device may pass the compliance stage, but fail to deliver the defined functionality and specifications to satisfy market needs. You may want to look for a supplier that has an ISO 13485:2016 with Design certification. This international standard specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements.
An efficient design interface will address healthcare regulatory policies. The design and development of medical devices is the most significant phase for the success of these devices. A loosely defined or designed product will not be compliant with regulations and in most cases, may not make it to the market.
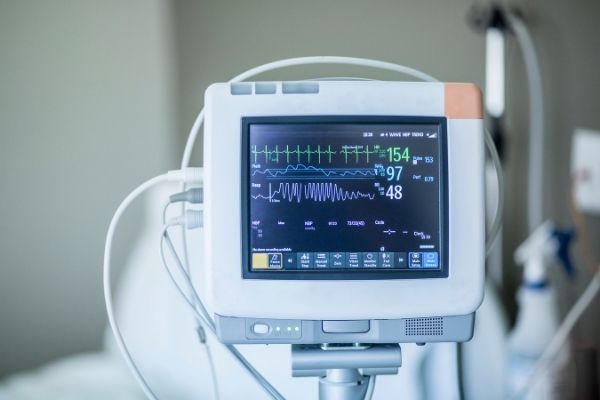
3. Aesthetically Appealing
Not only should products function well, but they should also be visually appealing, ergonomic, and intuitive to use. The right colors and graphics also impact the user experience. Is the unit used in bright light? Daylight? How about a recovery room at night? Having the right lighting and icons also affects the user experience. Consider if your products should be equipped with light sensors that enable the product to dim itself when exposed to changing light factors.
5. Simplifies the User Experience
A human interface design will simplify the user experience. No health care provider will create a product definition without patients in mind. User experience and satisfaction are always key. Medical professionals such as physicians and nurses can’t afford to fight with a machine that is complex or hard to understand.
When the human interface design process is followed, the end-product will make usage much simpler and more enjoyable.
6. Saves Lives
At the end of the day, companies in the healthcare industry are in the business of saving lives. Every product developed is created to directly or indirectly influence this outcome one way or another.
This is the most important part of the user interface for medical products—saving lives. But sometimes, medical devices with bad user interface design can do the opposite. Instead of being a lifesaver, they can cause more harm than good.
With all the above benefits, companies like Butler Technologies, Inc., whose focus is the design and manufacture of medical devices and instruments, is critical for successful user interface design. Now that you understand the benefits of good user interface design, here are the steps to make it happen:
The Best Process for Designing User Interface Products
Product Conceptualization
The right supplier will start the project by analyzing and identifying the demand and market to best formulate your idea into a plan. If you have an idea, the right supplier can help you conceptualize it and bring your vision to life.
Design Control Regulations
After identifying any hurdles, your supplier will follow guidelines set by regulatory bodies like the Food & Drug Administration (FDA), Health Canada, European Commission, and others. These regulations help ensure the final product is safe for end-users before the device goes into production.
Addressing Regulation and Compliance Needs
After considering the guidelines, the next step is to ensure that the product concept can be developed within the confines of the regulations. Before you begin design or production, the medical device will be taken through a rigorous exercise to ensure that it can pass certain regulatory compliance policies.
Testing and Validation
All medical devices must provide the functionality, reliability and usability objectives of their design. Aside from these, end users also want effectiveness and safety during use. Your supplier will build test units and perform testing that will ensure that the product will last and perform.
Risk Management
Sometimes, after a job completion report is submitted, a medical device designer and manufacturer must undertake risk management procedures on a regular basis. Risk management processes must also be consolidated into all stages of medical device design and development. This is a quality assurance control measure that can ensure the long-term safety and sustainability of the device.
Conclusion
To get ahead of your competitors and decrease time-to-market, you need help from an experienced medical device manufacturer. Butler Technologies, Inc. is a high-tech, precision screen printing firm that specializes in manufacturing printed electronics, printed heaters, biosensors, flexible hybrid electronics, membrane switches, graphic overlays, labeling and other user interface products.
Contact us today for assistance with your medical device design or manufacturing. We can help you create a product that meets the needs of your end-users and business. No matter what stage of development you’re currently at, we can help take your idea to market quickly and at an affordable price.